Abstract
Introduction: Treatment options for multiple myeloma (MM) have evolved in recent years, with introduction of novel therapeutic agents such as proteasome inhibitors (PI), anti-CD-38 monoclonal-antibodies (MoAb), and immunomodulatory drugs (IMiDs) that have significantly improved survival in patients with MM, however, MM remains an incurable disease characterized by a relapse-remission pattern. Despite advances in treatment, outcomes remain poor for those who are penta-RRMM (refractory to two different IMiDs: lenalidomide and pomalidomide, two different PI's: bortezomib and carfilzomib, and a CD-38 MoAb: daratumumab or isatuximab), with a historical median survival of 5.6 months. Recently B-cell maturation Antigen (BCMA)- directed therapy (BDT) is being increasingly used in heavily treated RRMM. This is a retrospective analysis, evaluating survival outcome of penta-RRMM pts who were treated with BDT (BCMA exposed) and comparing these outcomes with those who did not receive BDT (BCMA naïve)
Patients & Methods: Seventy-eight RRMM pts were identified as penta-RRMM at University of Kansas Health System between January 2015 to July 2022 and had electronic medical records (EMR) reviewed retrospectively. All patients identified met the inclusion criteria for penta-RRMM. Descriptive analyses were performed on available data obtained from patient characteristics. Survival curves were generated using the Kaplan-Maier method. Responses were evaluated using the International Myeloma Working group (IMWG) criteria. Baseline characteristics including high-risk cytogenetics, disease stage, lines of therapy (LOT), treatment response, and survival outcomes were obtained retrospectively from EMR. High-risk cytogenetics were defined as the presence of t(4;14), t(14;16) or del 17p; 1q21 gain or amplification; t(6;14); t(14;20).
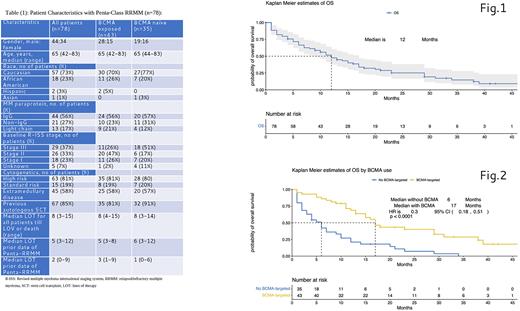
Results: The median age was 65 years, 44 (56%) patients had IgG isotype, 29 (37 %) had R-ISS stage III disease, 63 (81%) had high-risk cytogenetics, and 45 (58%) had extramedullary disease (EMD). The median number of previous lines of therapy for all patients was 8 (3-15), while the median LOT prior to reaching penta-refractory state was 5 (3-12), with a median of time from diagnosis to penta-refractory state 60 (4-176) months. Patient characteristics are summarized in Table 1. With a median follow-up (IQR) of 13 (4.9;29.5) months, median overall survival (OS) was 12 months (Figure 1).
Amongst this group of penta-RRMM patients 43 (55%) were identified as BCMA exposed vs 35 (45%) who were BCMA naïve. Type of BDT patients received included: belantamab mafadotin 15 (35%), Chimeric Antigen Receptor T cell therapy (CAR-T) 9 (21%), BCMA MoAb 6 (14%), and Bispecific T-cell engager (BiTE) 2 (5%). Eleven (25%) patients received more than one different BDT. The median time from initial diagnosis to penta refractory state in BCMA exposed vs BCMA naïve pts were 66 (4-176) and 48 (14-135) months, respectively. Median OS for BCMA exposed vs BCMA naïve pts were 17 vs 6 months, respectively (95% CI (0.18-0.51), p<0.0001). Common causes of mortality in all patients with penta-RRMM were disease progression, infection/sepsis, and secondary primary cancers in 89%, 6% and 5%, respectively.
Conclusion: Penta-RRMM patients have significantly worse outcomes, though our retrospective analysis indicated a significant survival benefit using BDT for penta-RRMM. Prospective studies are required to confirm these findings. Penta-RRMM represents a significant challenge with an unmet need for more therapeutic options.
Disclosures
Atrash:Celgene: Honoraria, Speakers Bureau; GSK: Honoraria, Research Funding; Janssen: Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; Sanofi: Honoraria, Speakers Bureau; Takeda: Honoraria; Amgen: Research Funding. Hashmi:JANSSEN: Consultancy; KARYOPHARM: Speakers Bureau; GSK: Speakers Bureau; Sanofi: Consultancy, Speakers Bureau; BMS: Consultancy. Mohan:Takeda: Research Funding; GSK: Research Funding; BMS/Celgene: Research Funding; Novartis: Research Funding. Alkharabsheh:National Community Oncology Dispensing Association, Inc: Consultancy; Genentech: Consultancy; Incyte: Consultancy; AstraZeneca: Consultancy; Agios: Consultancy; Amgen: Consultancy. Mahmoudjafari:Incyte: Membership on an entity's Board of Directors or advisory committees; Merk: Membership on an entity's Board of Directors or advisory committees; BMS: Membership on an entity's Board of Directors or advisory committees; Omeros: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal